 Let’s first define chemistry:
Let’s first define chemistry:
Chemistry is the science that deals with composition, structure and properties of matter.
- Composition is the amount and identity of the elements present.
- Structure represents the arrangement of atoms in a molecule.
- Matter is anything that occupies space and has mass.
- Mass is what represents matters resistance to change in motion. The problem with weighing things is that gravity is not constant, so we use mass.
Standard Units
The standard unit of mass is a kilogram: Almost exactly 1 liter of water. Eventually we will know it by the # of atoms in a KG.
The standard unit of length is the meter: Since 1983, the definition has been based on the speed of light. The distance light travels in 300 millionths of a second.
The standard unit of time is the second: Based on the waves of Cesium 133.
Temperature: Celsius although Fahrenheit and Kelvin are also used.
Metric prefixes
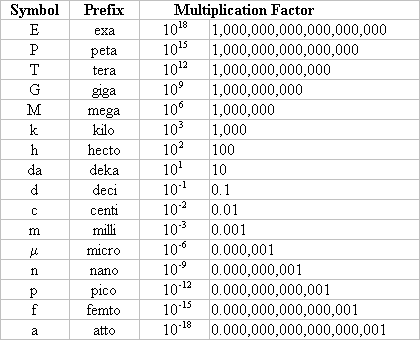
Out of 100 atoms on the periodic table, 1/3rd of them are important to us on a daily basis.
Physical properties are observations or measurements without altering its composition.
Chemical properties are observations made only by altering chemical composition.
Scientific Method
Observations – > Hypothesis -> Tests -> Results -> Law OR Theory
Law is the significant summary of observed behavior with no exceptions. The law of conservation of mass was the only law that had to be changed because Einstein came up with E=mc squared showing a relationship between mass and energy.
A theory is a model that provides an explanation. That’s not to say theories are not far reaching and significant though.
Theory -> Predictions -> Tests -> Results
If the results are consistent, that’s good. If the results are inconsistent, the theory needs to be modified.
Quantitative = measurement involved quantity. Qualitative= Observation involving quality or kind (not quantifiable) or descriptive adjectives that qualify what you mean.
Scientific notation
Scientific notation is made up of a ‘coefficient’ and ‘exponent term.’ as such:
- 5310 = 5.31 x 10^3
- 0.00189 = 1.89 x 10^-3
To multiply them together, you simply add the exponents:
(A x 10^B) (C x 10^D) = A x C x 10^(B+D)
To add scientific notation, the exponents need to be the same. Same if you are to divide them.
Errors
Random Error: inevitable variation.
Systemic error: directional
Precision: Closeness of multiple measurements
Accuracy: Cloesness of the average to the true or accepted value.
To minimize precision and accuracy errors, calibrate the instruments and take a lot of measurements.
Significant digits or figures: Going from left to right, count from the first non-zero # to the last non-zero #. Determine whether terminal zeros are significant by looking for a decimal or mark.
Density = Mass / Volume
| Solids | g/cm3 | Liquid | g/mL | Gases | g/L |
|---|---|---|---|---|---|
| ice | 0.92 | water | 1.00 | air (dry) | 1.29 |
| aluminum | 2.70 | olive oil | 0.92 | oxygen | 1.42 (1/5th air) |
| NaCl | 2.16 | Alcohol | 0.79 | Chlorine | 3.17 |
| Lead | 11.34 | Milk (diff kinds) | ~1.03 | Hydrogen | 0.084 |
| Wood | 0.3-0.5 | Mercury | 13.55 | Nitrogen | ~1.25 (4/5th air) |
Only ice has a much more lower density than liquid b/c of a very unique property called hydrogen bonding. Water is not coincidentally 1.00 g/mL. Water is defined that way because a definition of a gram was the mass of 1 cubic cm of water (1g = mass of 1 cm^3 of water).






