Pharmacology refers to drug administration (including dosage) and distribution in the body (pharmacokinetics). The area under the curve includes T half-life, peak concentration, time to achieve peak, elimination and protein binding. It’s one of the most important aspects of drug study, including antibiotics.
Peak and Trough
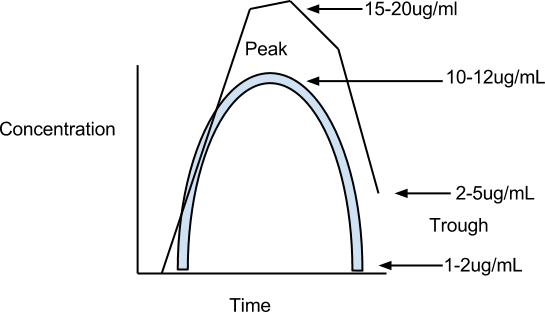
Let’s look at the peak and trough of gentamycin, which is an aminoglycoside. If the concentration is too high, the peak could lead to ototoxicity and nephrotoxicity. So it’s essential you measure peak and trough. If the peaks are exceeded, their middle ear apparatus are destroyed so they can’t tell their position and they could have kidney failure.
The normal peak and trough are 10-12ug/ml and 1-2ug/ml, respectively. The blue line shows the appropriate administered dose while the black line shows one that is excessive.
The route of administration is determined by the site of infection, the level of antibiotic required at that site, and the patients status and the urgency of appropriate treatment.
- Intravenous (IV) administration typically gives the highest concentrations in the shortest time and is thus preferred for seriously ill patients
- Intramuscular (IM) administration produces lower concentration levels over a longer period than IV but a higher concentration than oral administration.
- Oral administration is beneficial, dependent on the infection, for maintenance doses.
- Other methods of administration include sublingual, mucocutaneous (such as eye, nose, ear) and topical.
For seriously ill patient with SIRS and a gram positive gram stain from the C&S, what route of antibiotic administration do you recommend?
IV because it’s the fastest possible.
Bioavailability affects the concentration of availability of the active drug in the systemic circulation because the drug must succumb to the gastrointestinal tract. It’s also influenced by solubility, which relates to the excipients (vehicle; tablets and capsules for oral, suppositories for rectal) in which the material is in. In regards to the question of generics, the FDA regulates the generics so they are the same as the proprietary versions (at least for those manufactured in the USA).
An example of bioavailability are the Fluoroquinolones (Ciprofloxacin, Levofloxacin and so forth). It’s recommended that you should avoid taking it 2 hours before a meal or 4 hours after a meal and also avoid antacids.
LOOK AT PAGE 493 FIGURE 21.3.
Drug distribution in body tissues should be considered in pharmacology/pharmacokinetics. IM/IV/Oral, it doesn’t matter, the drugs all get to the blood/systemic circulation. Even the nasal cavities are highly vascular and it has been shown (chinese showed this for centuries, long before jenner formulated it). Sometimes, you want the drug to be concentrated in the lungs sometimes, but with a pill taken by mouth, it has to cross into the blood stream. If it’s bound to protein, it can’t cross because the sheer size of it prohibits crossing. This is something the drug manufacturer has to document that protein binding is not significant or there is some mechanism where the drug can be freed by the protein.
If the drug binds to protein it will not cross into the tissue. (In other words, protein-bind = cannot cross)
Not protein bound (unbound drug) means it crosses by…
- dissolving in lipid (lipid soluble drugs) because the cell membranes are made of lipid.
- protein pores which are transport proteins
- means of enzyme catalyzed transport
One of the quintessential antifungals: Amphotericin B, used widely for systemic fungal infections, is extremely toxic. And why would that be an issue? Both fungi and your cells are eukaryotic. In the business this is known as amphoterrible. Some patients rather die than take it because if they do take it they get this metallic taste in their mouth, persistent ear ringing, nausea, upset GI and vomiting.
Part of the toxicity can be overcome and reduced by targeting delivery. Amphotericin B is now encapsulated in liposomes (now known by the market name: ambisome). A liposome is a fat globule (lip=lipid=fat), and inside of it is amphotericin B. It will dissolve in the lipid membrane due to the similar structure and that will ensure that the delivery to the tissues is much more facile rather than circulating ad libitum (freely) throughout the body. Amphotericin B is now marketed as Ambisome because it’s in this lipid vesicle.
Reference Page 494: Read “Drug Distribution” section to get a fuller idea.
Ciprofloxacin has an extended preparation of cipro. There are several coatings so there’s an extended release of the drug (by dissolving the layers slowly), that allows a different dosing regimen.
Spectrum of activity
Antibiotic: Only refers to drugs that are effective against bacteria.
Antimicrobial: Includes antiparasitic, antifungal, antiviral. An antiviral drug is commonly referred to as an antibiotic but it’s not an antibiotic.
The range refers to the number and variety of organisms that can be covered. When we talk about any antimicrobial agent, we talk about whether it’s a broad spectrum (covers a wide range of organisms) or narrow spectrum (covers a narrow range of organisms; generally one type of bacteria).
Turn to page 495, figure 21.5: YOU MUST KNOW THIS CHART. Know which antibiotic is used against what type of bacteria
Pencillin: When asked for a narrow spectrum antibiotic on the test, DO NOT say penicillin, as it can be used against outside of its spectrum of gram positive activity, even though the table in the book says it’s a narrow spectrum. Ampicillin is a variation of penicillin. Up to 15-20 years ago, penicillin was the treatment of choice for N. gonorrhoea and it’s not anymore due to resistance.
Isoniazid/Ethambutol: Narrow spectrum, effective against mycobacteria.
Polymyxin B: Narrow spectrum: effective against gram negative bacteria.
Broad spectrum:
- Ampicillin (covers gram positive and gram negative)
- Chloramphenicol/tetraycycline (covers everything except mycobacteria)
- Streptomycin (covers everything except gram positive)
What is the significance of using broad versus narrow?
1. Broad spectrum gives greater perturbations to normal flora so it leads to secondary infections (pseudomembranous colitis; antibiotic associated colitis).
2. The possibility of resistance will be increased with broad spectrum because it’s used against a wider variety of organisms. In other words, the chances of resistance developing are more likely with the broad spectrum antibiotic because it covers such a wide range of organisms so there’s a greater potential for resistance.
Some antibiotics are classically associated with this: Clindomycin, for example, in the early days had an unsavory reputation for an increased incidence for pseudomembranous colitis and it was particularly useful against anaerobic bacteria. The broader the spectrum, the more likely it is to perturb normal flora.
You will be asked a question on fecal transplant (aka fecal bacteriotherapy):
- Fecal transplant restores the colonic flora by introducing healthy bacteria.
- The procedure involves single to multiple infusions (by enema, colonoscope, etc) of bacterial fecal flora from a healthy donor, into a patient suffering from Clostridium difficile infection (pseudomembranous colitis; antibiotic associated colitis).
Resistance to Antibiotics/Antimicrobial agents
Factors influencing resistance:
- Misuse
- Medical “Malpractice” (overuse). Not deliberate but an overuse.
- Cross border / Internet access / International access without prescription.
- Questionable formulations(preparation offshore) / Counterfeits
- Self medication
- Rampant & aggressive advertising by “BIG pharma.”
- Increased patient demand and access.
- FDA pressures. (Not so common with antibiotics but rich young white professionals, like doctors, lawyers, pressure the FDA to fast track the medications w/o adequate research and the data may be soft.)
- Antibiotic residues in meat/fish. Xenobiotics can be hormonal-like, and in some countries like Brazil you have early onset of puberty.
- Animal husbandry. (supplementing cattle, feed with antibiotics & hormones..etc. Cattle put on more weight and they can make more money)
- Poor patient compliance (Premature discontinuation or missing a dose; Certain levels are required for efficacy)
- Access and availability
- Insurance coverage, which also affects availability. (It’s not your doctor that decides what drug you get, it’s your insurance). A social disaster.
- Migration / International Travel / Offshore medical treatments. The latest denizen on the block, Extended spectrum organisms.
- Medical tourism (TNA: titties and ass in India).
- Continued adaptive responses, mutations, changes in the target organism.
Bacteria can adapt so quickly to changes, it surpasses anything we are capable of. Every single antibiotic discovered to date has been shown to be associated with resistance in target organisms, even in circumstances where there is no evidence of prior exposure.
Since bacteria have such a wonderful adaptive capability and can multiply so rapidly, that one in a million or billion chance creates naturally occurring resistant organisms. You start off with a mixed population of resistant and susceptible organisms. You treat with antibiotics and the susceptible ones will be eliminated and you’re left with a resistant population which will multiply like crazy and that drug no longer become effective.
For this reason, the CDC launched an antibiotic tracking system for hospitals. Antibiotic use can lead to antibiotic resistance which can increase the risk of hospitalization or lead to treatment failure in sick patients, according to the CDC.
Antibiotic-resistant infections are likely to require longer and more costly hospital stays and have higher mortality rates. If first-line drugs don’t clear up the infection, second or third-line drugs may be even less effective, more toxic, and more expensive.
Real threat of returning to a pre-antibiotic era: Drugs no longer useful because organisms are resistant to everything we have come up with.
With every single antibiotic noted to date, within 6 months of use, antibiotic resistance has been documented. So there’s no antibiotic which bacteria have not developed resistance. It’s microbial survival of the fittest.
*T/F Question! – Emerging resistance to N. Gonnorhea is developing. (true)
Basic mechanisms of resistance
One single organism may have several different mechanisms to several drugs. The bacteria can either destroy the drug or alter the drug or inhibit the drug or do all of them.
Reference: pg. 496 Table 21.2
This isn’t an exhaustive list, there’s many variations of each theme. Know the 3 primary mechanisms and one antibiotic associated with each one. These are all ways that bacteria can destroy/alter/inhibit the drug (antibiotic).
- Destroys. There are several ways.
- Plasmids can encode enzymes that chemically alter aminoglycosides (by acetylation or phosphorlyation)
- Plasmids can encode beta-lactamase which inactivate beta-lactam antibiotics such as penicillin and cephalosporins.
- Alters drug.
- An aminoglycoside normally tries to bind to a 30S ribosome subunit, so as a resistance mechanism, the bacteria makes a different, altered ribosome that won’t allow the drug to bind to it and won’t be inhibited.
- Quinolone normally tries to bind to DNA topoisomerase to inhibit DNA synthesis but the bacteria could make an altered DNA topoisomerase so the antibiotic won’t bind to it.
- Rifampin normally binds to RNA polymerase to inhibit RNA synthesis but the bacteria makes an altered polymerase so it won’t bind to the drug.
- Inhibits drug entry or removes drug.
- Penicillin binds to proteins in the outermembrane to enter the cell and block peptidoglycan synthesis, but the bacteria can change the shape of these porin proteins so the drug can’t enter.
- Erythromycin and tetracycline normally bind to ribosome subunits to inhibit protein synthesis, but the bacteria creates a new membrane transport system to literally pump the drug out of the cell.
Increasing the number of different mechanisms of resistance increases the likelihood of resistance.
T/F Question – Bacteria are conscious or sentient entities. (true)
What evidence is there that bacteria are conscious or sentient entities?
Capsule, spore , nutrition, temperature, adaptive, listeria cold enrichment.
- Capsule formation is determined on nutrient availability. Bacteria respond to nutrient excess by making a capsule which is protective and allows them to attach to tissues to produce infection.
- Spore formation and germination are dependent/determined by environment. When nutrients are limited and water is not available, they will form spores. When conditions are favorable, they will germinate.
- Motility associated with flagella. Taxis (movement) is a directional response. Phototaxis. Magnetotaxis. These are all responses to various stimuli which means they need to be coordinated to move in a certain direction.
- Quorum sensing (we talked about this with biofilms!)
Evaluation of Efficacy
How do you know if antibiotics are going to work? It’s the S in C&S (Sensitivity).
Susceptibility testing (for lab practicum).
- Kirby Bauer Disc diffusion
- R – not effective
- I – when there’s evidence of accumulation of organism in blood/bodily fluids.
- S – use
- MIC, MBC -> Broth Dilution
- Agar Dilution
- “E” Test
Question on exam You have a plate with antibiotics, and you look for the zone of inhibition. This zone is arbitrary and has no significance until you refer to the NCLS Chart. If the chart says that 12mm or more is equivalent to susceptibility, whereas 10 or less is resistant, and you have 12 or more, it means it’s susceptible and so forth
If control zones are not within acceptable limits or if the identity of the organism is unknown, the data from the test is invalid and must be discarded necessitating that the test be repeated.
A high M.I.C. (minimum inhibition concentration) typically indicates resistance. (on the KB chart) I’ll give you zone sizes, the results of the test and ask you whether it’s R or S or I
Now turn to pg. 500 – dilution of antibiotics.
You have different graded concentrations, all tubes inoculated with the same organism. The tubes with no antibiotics are control. If the control tubes have no growth, they are dead. (HE WILL ASK THIS). 2 mg/mL has no growth, so the MIC is the lowest concentration that inhibits the growth of the bacteria. I could give you the results like this or a chart and ask you where the MIC is.
To determine the bactericidal concentration, take all the negative tubes and put them on plates. If they grow, they are not totally inhibited. The MBC in this case is 8 mg/ml. The MBC is the minimum bactericidal concentration: the lowest concentration that KILLS the organism, established by taking cultures with no obvious growth, to antibiotic-free media and looking for growth. If the subculture in the antibiotic-free media grows, the bacteria were inhibited but not killed; The MBC is equated by those negative cultures that do not grow on the antibiotic-free media, documenting that the bacteria were killed.
Uses/Applications of MIC/MBC Data: This is a guide to the choice of therapy.
1. If the MBC 64x times greater than the MIC, that indicates TOLERANCE which means the antibiotic is not likely to be effective for treatment. You have to use so much of the antibiotic to kill the organism that you exceed the therapeutic ratio or range and you can harm the patient.
2. Efficacy is mandatory data for FDA approval and essential documentation.
3. Comparative efficacy. In other words, the FDA is not necessarily concerned with proving just another drug without a significant advantage, unless it’s a generic, which the data would have been established by the proprietary brand name. So this is one of the major planks in the approval process so they have to demonstrate that the drug actually works.
4. Even more importantly, it monitors the development of resistance, or changes in susceptibility patterns/response.
You’ll typically see:
MIC50 means ½ of the isolates tested are inhibited (which means they are susceptible).
MIC90 means 90% (9/10) are inhibited.
MBC50 means ½ of the isolates are killed by the concentration.
MBC90 means 90% of the isolates are killed by the concentration.
YOU’LL NEVER SEE MBC 100. There’s always going to be an organism that is going to be resistant, given the fact that antibiotic resistant can be documented without prior exposure and within 6 months of introduction of any antibiotic.
If you require an increase of concentration, it’s indicative of an increase in resistance, you need a greater concentration of antibiotics.
↑ [C] = ↑[R] (C=concentration, R=resistance)
In the year 2000, MIC50 = 1ug/ml
In the year 2010, MIC50 = 5ug/ml
Let’s look at ciprofloxacin… If an effective dose is achieved at 5ug/ml, and you have MIC50 but years down the line 50% of your isolates may require 10ug/ml. If you have an organism that requires TWICE the concentration to be inhibited, it means 5ug/ml is not going to be effective anymore. You can’t just double the dose cause then you have toxicity.
Be familiar with why an organism showing tolerance is not effective to that treatment.
Agar dilution: Rather than having a bunch of tubes for the MIC/MBC, the tubes are replaced by agar, with varying concentrations of antibiotic or antimicrobial agent.
Neutropenics. The big problem is neutropenics are treated with antifungal treatments like Nystatin Px but a lot of those patients end up with fatal fungal infections. So the question is… are the deaths due to antifungal failure? In other words, were the organisms resistant to nystatin? The failure of treatment was not due to the resistance of the organisms.. none of the fungi were resistance. The failure was due to the neutrophil count not being increased.
Fungi are not tested routinely for susceptibilities, although several experts are recommending that this be done. For the most part, the responses are predictable. What is done instead is the tests are done in pools of isolates and they are periodically tested with the agar dilution or now they have modifications with respect to instrumentations that allow you to detect growth dependent on the concentrations
E test
The E test is not as widely accepted in America because here’s a bit of jingoism since it wasn’t developed in the USA. It’s slowly gaining acceptance and becoming more routine now.
The E test is useful because you have a lawn of bacteria and you could put several different antibiotic strips that could be vancomycin, penicillin, gentamycin, tobramycin. Whoever came up with this is certifiably genius.
The concentrations of the antibiotics are layered sequentially and sold commercially. You put this strip on the lawn and you end with growth or no growth or a zone of inhibition.
The point of intersection where the point of inhibition coincides with the level marked on the strip is the MIC. The beauty of this is you could test 4-5 different antibiotics separately and you could get break-point MICs. You will indubitably get questions on the exams with this.

The E test, a gradient diffusion method that determines antibiotic sensitivity and estimates minimal inhibitory concentration. The plastic strip, which is placed on an agar surface inoculated with test bacteria, contains an increasing gradient of the antibiotic. The MIC in ug/ml is clearly shown.
Sites of Action and Examples of Antibiotics Targeting PROKARYOTES
Reference page 501, figure 21.10
You see prokaryotes and eukaryotes. There’s a clinically significant difference between the two. This is a prime example. Not only do these differences reflect specificity of action but explain the toxicity associated with antimicrobial agents.
How is toxicity of antibiotics and adverse drug reactions related to the differences of eukaryotes and prokaryotes?
The specificity of microbial action is related to the structural, physiological and metabolic differences that differentiate microbes from the human body.
You must be familiar with ALL of these sites and the relevant antibiotics that target those specific sites. Any part of the bacteria is fair-game.
What antibiotics inhibit the bacterial cell wall?
- Penicillin
- Cephalosporins
- Vancomycin
- Bacitracin (used topically as an antibiotic).
Read the “Cell Wall” paragraph. You only find peptidoglycan in bacteria. These agents have no effect on eukaryotic cells because all eukaryotic cells lack peptidoglycan.
Question on test: What is the treatment of choice for L forms and why? An agent that inhibits protein synthesis (gentamycin) and penicillin that inhibits the reformation of the cell wall. Some L forms can reform the wall, so if they reform the wall, you use penicillin to inhibit the cell wall and the gentamycin to inhibit protein synthesis, so that’s a synergistic example of antibiotics. (Synergy = 1+1=11).
Beta-lactam ring -> Penicillinase (beta-lactamase) -> Produces penicilloic acid, which inactivates the penicillin. The penicilloic acid is also a product that can trigger allergic/hypersensitive reactions.
What antibiotics inhibit the bacterial cell membrane?
- Polymyxin B. You don’t use it as a widespread use of an antibiotic. It’s used topically. Read “Cell Membranes” paragraph. ALL cells have membranes so it’s not an ideal target of drug action but there are some differences between microbial and mammalian cell membranes. Most drugs that target cell membranes are highly toxic when administered systemically.
- What is the basis of specificity of polymyxin? The eukaryotic cell membrane has a different sterol composition that isn’t recognized as well by the polymyxin.
What antibiotics inhibit the Nucleic Acid synthesis?
- Rifampin (RNA)
- Quinolones (DNA)
- FQ = Fluoroquinolones. ie.,
- Ciprofloxacin
- Levofloxacin
- Temafloxacin
- When you see FLOXACIN in the drug word, that’s a Fluoroquinolone and they inhibit DNA synthesis
- What is the basis of action of FQ’s? How do they act? They inhibit an enzyme that is central to DNA replication that initiates and causes negative super coiling to the DNA prior to splitting by helicase, called DNA gyrase aka topoisomerase. Both human and bacteria require DNA gyrase but there’s Type II topoisomerase that’s found in prokaryotes. The bacterial DNA gyrase (enzyme) differs from the eukaryotic DNA gyrase.
- The stupid way I remember fluoroquinolones inhibits TOPOISOMERASE activity… is that they are both big words with many syllables!
- The FQ is going to preferentially bind to the bacterial enzyme to a much lesser extent than the human gyrase.
- The major adverse effect of FQ is arthropathy (joint pain), primarily associated with a principal weight bearing joint because it inhibits the conversion of cartilage to bone. That is why children are most susceptible to this adverse effect but adults will get tendonitis, tendon rupture, bursitis as well.
- Another adverse effect is that it causes photosensitivity and photomutagenesis which are light-induced changes to DNA.
- FQ = Fluoroquinolones. ie.,
The product monograph is a quasilegal guideline document. As a patient you might get a package insert that gives you the adverse side effects and how you should take the dose and whatever else. First thing is shows is the warning. FQ’s are associated with an increased risk of tendinitis and tendon rupture (Is your ACL a tendon or ligament? It’s a ligament. Tendons are muscle to bone. What are ligaments? Bone to bone connective tissue. What’s the similarity? They’re both dense connective tissue).
- Pharmacology/Pharmakinetics tells you how the drug is distributed throughout the body which tells you the efficacy. The binding of cipro to serum proteins is 20-40% which isn’t likely to be high enough to cause significant protein binding interactions w/ other drugs.
What antibiotics inhibit Protein synthesis?
- Aminoglycosides (Proteins are made of amino acids, so aminoglycosides inhibit protein synthesis)
- Tobramycin
- Gentamycin
- Where have we seen gentamycin mentioned before?
- Gentamycin + Penicillin = synergistic effect against L forms.
- Gentamycin peak and trough = 10-12ug/ml and 1-2ug/ml
- peak is reached in 2 hours, trough in 4 hours. (symmetrical)
- side effects if too much: ototoxicity and nephrotoxicity
These antibiotics require monitoring. The therapeutic acceptable peak levels of these are 10-12ug/ml and the trough is 1-2ug/ml. The peak is usually 2 hours and the trough is 4 hours after administration. Peak and trough levels are a guide to the effective levels which are not to be exceeded to avoid toxicity. This is particularly important in patients with deficient kidney function. What would you do if levels exceeded peak and trough? You could lengthen the dosage frequency or lower the dose but you can’t arbitrarily do that because you have to consider the concentration achieved at the site of infection. If you need 5ug/ml in the CSF and you’re only getting 2ug/ml, is it going to be effective? NO. So you need to consider MIC, MBC and the concentration achieved at the site of infection.
Side effects: Ototoxicity and nephrotoxicity (middle ear damage and kidney damage) are the two primary consequences of aminoglycoside if peak and trough levels are exceeded, especially in elderly.
- Chloramphenicol (page 509, figure 21.14)
- Chloramphenicol is a BROAD SPECTRUM antibiotic! (review page 495 figure)
- In rare cases, chloramphenicol is fatal because it can cause aplastic anemia (the bone marrow completely stops producing blood cells). This happens in 1 out of 30,000 drug recipients and is inevitably fatal.
- Another threat is gray baby syndrome because newborns may die from multiple toxic complications so this drug is ONLY given to extremely ill hospitalized patients.
- Tetracyclines
- Erythromycin
What antibiotics inhibit Folic Acid Synthesis? (page 502)
- sulfonamides
- trimethoprim/sulfamethoxazole
In this case we are looking at metabolism. FA is a precursor to the biosynthesis of nucleic acids. Trimethoprim blocks the pathway in which folic acid is converted into nucleic acids in bacteria but not in humans because humans use a different pathway to utilize that folic acid.
UTI: One of the most widely used antibiotics for treatment of UTI. Where do the bacteria primarily come from that cause UTI? The area between the genitalia and the rectum. Also, honeymoon cystitis is excessive sexual intercourse which predisposes to UTI.
Cranberry juice does not treat UTI’s, it reduces the incidence rate.
Pneumocystosis / Pneumocystic pneumonia is a form of pneumonia caused by a yeast-like fungus.
Sites of Action and Examples of Antibiotics Targeting EUKARYOTES (Fungi, Protozoa, Worms)
Folic acid synthesis
- trimethoprim (same as prokaryotes)
Nucleic acid synthesis
- flucytosine (RNA) an anti-fungal agent (coincidentally fluoroquinolones inhibit nucleic acid synthesis [DNA though] in prokaryotes, they both start with fl… hopefully that helps)
Cell membrane:
- amphotericin B
- imidazoles
- nystatin
Antifungal drugs (pg 511)
- nystatin
- amphotericin b
- imidazole and triazole
- flucytosine
- griseofulvin
Antiparasitic drugs (pg 512)
- mebendazole
- metronidazole
In Table 21.5: Remember Malarone for Malaria:
Malarone is the synergistic combination of 2 drugs: atovaquone and proguanil (he will probably ask this!)
Antiviral drugs (page 514)
- Acyclovir inhibits viral DNA
- Ribavirin inhibits viral RNA
Know how Acyclovir works!
Acyclovir is structurally similar to the nucleotide guanosine. An enzyme, found only in virus-infected cells converts acyclovir to acyclovir triphosphate, the actual antiviral drug. This false nucleotide competes with GTP to incorporate into DNA, leading to chain termination and stops DNA replication.
REVIEW
What is the major contraindication associated with antibiotic use? An indication is a circumstance of an infectious disease with characteristic etiology that warrants use of an antibiotic. Contraindications preclude to negate indications.
1. A history of allergic response or hypersensitivity is a contraindication, including anaphylactic shock which can be fatal.
2. Synergy: an amplified effect beyond an additive. The sum of the parts are more effective than their individual selves (1 plus 1 = 11). (malarone = atovaquone and proguanil synergistic combination)
Toxic effects of fluoroquinolones?
Arthrotopy, rupture of the ACL, tendinitis, bursitis, photosensitivity, photo-mutagenicity. The major problem is arthrotopy in every age group but particularly children. While you’re on fluoroquinolones, you have to avoid direct light.
———————
ESBL = Extended spectrum beta-lactamase bacteria (beta lactamase 2.0)
With ESBL’s, if the organism is resistant to penicillin then it’s going to be resistance to beta lactam antibiotics because it means it has beta lactamase.
“The devilishly clever bacteria met our challenge by creating beta lactamase, an enzyme that grants many bacteria immunity to penicillin-type antibiotics. In turn, we upped the ante by developing new kinds of antibiotics that trounced these beta lactamase-producing pathogens.
But the bacteria weren’t done yet. Some tricky little bugs had a trick up their metaphorical sleeves: Beta Lactamase model 2.0, known to us as extended-spectrum beta lactamase, or ESBL. This enzyme not only chops apart penicillins, but cephalosporin antibiotics, too (all of the antibiotics whose generic names begin with “Cef-”).”
Page 278… Table 1.4, Know the problems associated with atypical bacteria.
The part about chlamydia.. they are atypical because they don’t have a cell wall … you can’t grow chlamydia on blood agar, it is an obligate intracellular pathogen so it must grow on tissue culture.
What are the 3 species of chlamydia that infect humans? Chlamydia pneumoniae, trachomatis (blindness, STD), and psittaci (causes ornithosis or psittacosis; parrot fever)
Chlamydia pneumonia. What is the primary significance? It causes pneumonia! Secondary significance: Coronary artery disease; myocardial infarction due to high antibody levels. People who have had heart attacks have high levels of antibodies which means they have been exposed to the bacteria.
Rickettsia: What is the only rickettsia that is able to survive outside the living host? Coxiella burnetii is the only rickettsia that could survive outside of the living host. It forms a spore like entity which isn’t equal to the bacterial endospore but can survive in soil, feces, etc for an extended period of time. It’s the only rickettsia that can do that and the etiology of Q fever.
Microscopy: Know the limits of resolution, the various types of microscopes (brightfield is the most common, electron microscopy is used for viruses, fluorescent is used for identifying antigens/antibodies and you don’t need isolation in pure culture to do fluorescent marking).
- Human eye: 200 micrometers.
- Brightfield: 200 nanometers (0.2 micrometer)
- Scanning: 10-20 nanometers
- Transmission = 1-2 nanometers.
You will be asked something like: How many times smaller of an object can be seen with a scanning electron microscope than the naked eye? Convert everything to nanometers to find out.
Review previous tests. Review those god damned presentation sheets. Review fucking everything. Good luck.






