Anabolic and Catabolic Reactions

Let’s begin with the cell theory. The cell theory is that the basic structural and functional unit of life is a cell. Each of you began as a cell from a zygote. Even a whale starts as a single microscopic cell. From that original zygote you just increase the number of cells.
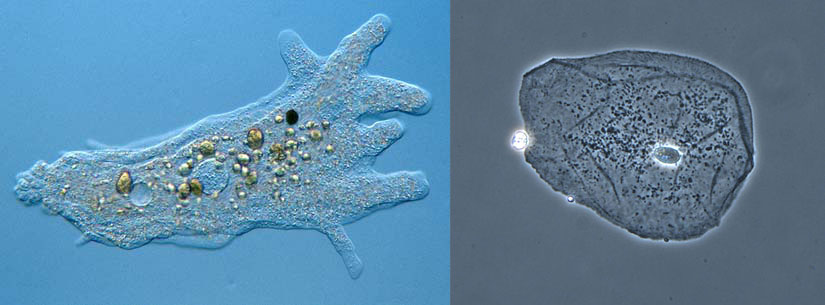
An amoeba is a single celled protozoa that lives in pondwater. The rounder cell on the right is a typical human cell taken from inside someones cheek. Despite how drastically different they look and the different environments they live in, there are a lot of similarities between all living cells. (1) For example, they both have cell membranes. The cytoplasm on the inside of any cell is about 80% water. Since most of the stuff inside is water, it’s also called Intracellular Fluid (ICF). (2) All living cells live in a water environment. The water environment the amoeba lives in is called pondwater. The fluid that surrounds human cells are called tissue fluid. Since this is water outside the cell, this is known as Extracellular Fluid (ECF). In fact, if you scrape the top dead skin cells off of your skin, some white fluid will start to come out.
Two Major Types of Metabolic (Biochemical) Reactions: Anabolic and Catabolic
Let’s talk about two major types of metabolic reactions. The word metabolic means biochemical. The two types are called anabolic and catabolic.
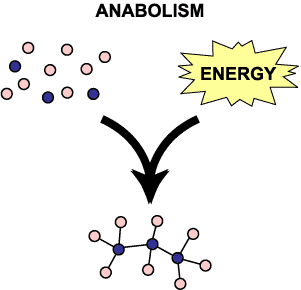
Anabolic reactions are associated with growth. Catabolic are associated with the release of energy and energy production. At any moment both reactions are occurring. Collectively all of these reactions are called your metabolism.
Anabolic reactions are basically taking what’s in your food and forming large complex molecules. The context you’ve all heard anabolic before in is that athletes will take anabolic steroids for growth. When sugars are joined together to create glycogen, that’s anabolism. When you join amino acids to make proteins, like in your muscles, that’s an anabolic reaction. When fatty acids in your food are joined to form a triglyceride, that’s an anabolic reaction.
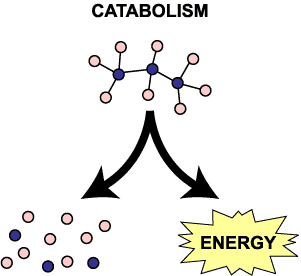 Catabolic reactions are the breakdown of organic molecules for energy. Breaking down sugars for energy, fat for energy, and so forth are all catabolic. To remember what catabolic means, think a CATastrophe where things are falling apart and breaking apart. You could also think of CATS that tear apart your furniture.
Catabolic reactions are the breakdown of organic molecules for energy. Breaking down sugars for energy, fat for energy, and so forth are all catabolic. To remember what catabolic means, think a CATastrophe where things are falling apart and breaking apart. You could also think of CATS that tear apart your furniture.
Maintaining Body Weight
If you are an adult, this is however tall you’re going to get. None of us are growing any taller. There is only one direction we can grow and that is wider. Since most of us don’t really want to grow any wider that means we must breakdown molecules in our body as fast as we make them or else we will get fat. Even a body builder that increases proteins in their body cells will never grow taller, but wider. If we want to get thinner, we have to slow down the growth reactions. How? Eat less. The way you form these organic molecules are from the food. Eating less slows down the anabolic reactions. If you do things that require energy, such as exercise, that speeds up the breakdown of fats and carbs for energy and speeds up the catabolic reactions. If you want to get thinner, eat less and exercise. Exercise speeds up the catabolic reactions. If we, as adults maintain a balance between anabolic and catabolic we stay the same weight.
For children, these reactions should not be in balance. In a child, the anabolic reactions have to be greater than the catabolic. Boys start their growth spurt after girls. That’s why when they are in 7th or 8th grade, the girls are still taller than the boys. The boys change a couple years after the girls. Guys may gain 2-3 inches in that growth spurt between 14-17 years of age. During that growing period they will eat up all the food in your refrigerator. They are growing like crazy during that time. But what happens to both boys and girls at 18-19 is that if they keep eating the same way, they won’t grow taller anymore but only wider. This is the phenomena everybody notices.
Two Types of Anabolic Reactions
1. Dehydration Synthesis Reactions
Anabolic reactions involve the joining of smaller molecules together to form larger, more complex molecules. This occurs through dehydration synthesis reactions. These are the most common ways smaller organic molecules can be formed into more complex ones and applies to the formation of carbs, proteins, lipis and nucleic acids.
Remember when we explained how margarine is made? You start with polyunsaturated vegetable oils and hydrogenate them so that it looks like a saturated fat like those found in animals. We didn’t call it this before, but that’s a reduction reaction because it involves adding hydrogens and electrons to a molecule so that it has more calories of energy.
2. Reduction Reaction. (“RIG”)
A reduction reaction involves the adding of hydrogens and electrons to a molecule. Whenever you do that, it gains calories of energy because when you split a hydrocarbon bond, it releases energy.
Incidentally, the mnemonic for remembering this is…
OIL RIG: Oxidation Is a Loss (of H+ and e–), Reduction Is a Gain (of H+ and e–)
Two Types of Catabolic Reactions
1. Hydrolysis Reactions
Catabolism is the breaking apart of molecules to smaller molecules to release energy. An example of a catabolic reaction is digestion and cellular respiration where you break apart sugars and fats for energy. Hydrolysis is the way in which this is done and it is basically the reverse of a dehydration reaction. Breaking down a protein into amino acids or a triglyceride into fatty acids or a disaccharide into monosaccharides are all hydrolysis or catabolic reactions.
2. Oxidation reactions (“OIL”)
Oxidation reactions involve the removal of hydrogens and electrons from an organic molecule.
Antioxidants
Since we just talked about oxidation reactions, let’s go over what antioxidants are.
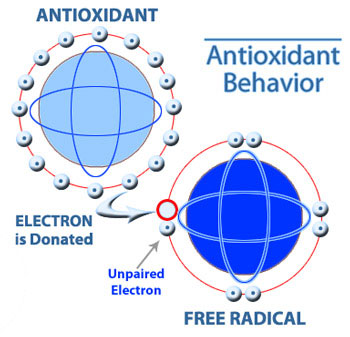
There are these molecules called free radicals that oxidize, or in other words, strip hydrogens and electrons off complex organic molecules. It appears that when free radicals do this, they accelerate the aging of cells and may also cause normal cells to transform into cancer cells.
An antioxidant prevents free radicals from stripping off hydrogens, illustrated to the right. For example, Vitamin C and Vitamin E are antioxidants. So the point of taking antioxidants or eating food that contains lots of them is to help slow down aging and prevent cancer from forming.
Cellular respiration
- Anabolic and Catabolic Reactions
- Intro to Cellular Respiration: The Production of ATP
- How Glucose Levels are Regulated in the Blood Stream
- Cell Respiration Part 1: Anaerobic Respiration (Glycolysis and Fermentation)
- Cell Respiration Part 2: Aerobic Respiration (Transition Reaction & Kreb’s Citric Acid Cycle)
- Cell Respiration Part 3: Aerobic Respiration (Electron Transport System)
- The Catabolism of Fats and Proteins for Energy
- The Catabolism of Nucleic Acids
- Oxygen Debt






