The photon’s energy would be associated with its frequency (nu), through a proportionality constant known as Planck’s constant (h), or alternately, using the wavelength (lambda) and the speed of light (c):
- E= Photon energy
- ν = Frequency (per s, s-1)
- λ = wavelength
- h = Planck’s constant = 6.62606957 × 10−34 J·s
- c = speed of light = 299,792,458 m/s
Planck–Einstein equation
E = h • ν
Since the frequency ν, wavelength λ, and speed of light c are related by c = λν, the Planck relation can also be expressed as:
E = hc / λ
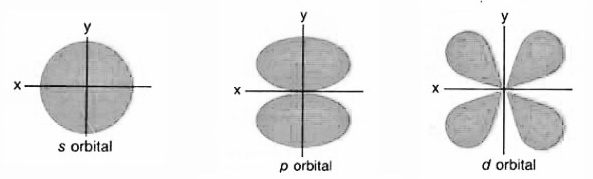
Orbitals
- s-type, set of 1
- P-model, set of 3
- d-type, sets of 5
- f-type, sets of 7
Aufban Principle
1. Principal Quantum number (PQ #) increases with energy: 1,2,3,4,5,6,7…
2. Energy increases with orbital type: s, p, d, f…
3. Type numbers: sets of 1 , 3, 5, 7 (associated with s, p, d, f orbitals)
4. Electron spin: There’s 2 e- per orbital but to do that they have to have opposite spins. ^ or v
Shells (PQ #)
As you add shells, they move further away from the nucleus but each shells moves less further away with each subsequent shell. The shortest wavelength has the highest energy. And the valence e- are the ones in the outer shell.
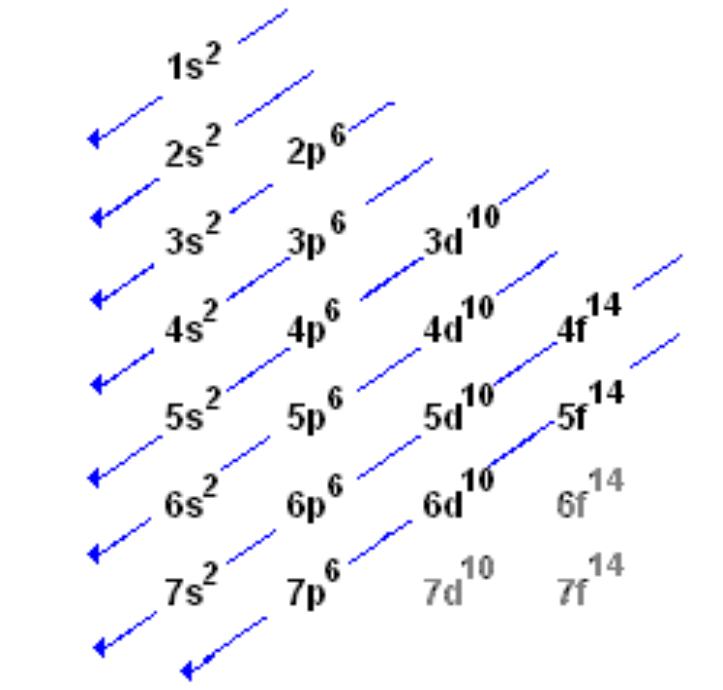
There is an energy overlap between 3p6 and 4s2. 4s has less energy than 3d:
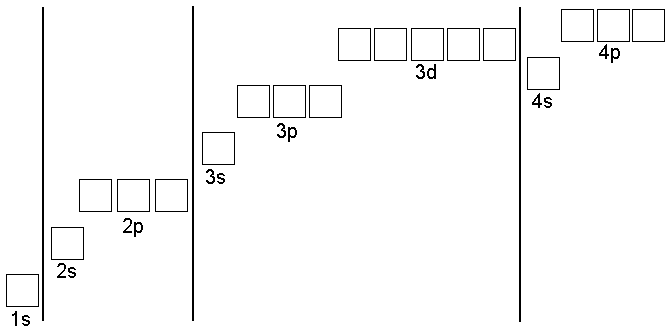
Electron configuration
Transitional metals have similar properties because their e- configuration is similar.
Examples:
- Li: 3e = 1s2 2s1
- C: 5e = 1s2 2s2 2p2
- Na 11e = 1s2 2s2 2p6 3s1
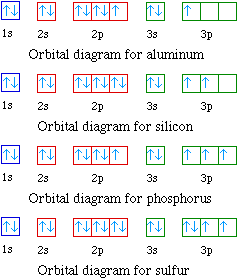
Orbital Diagram
Another way to identify the location of each electron is via an orbital diagram. The sets with only one electron indicate potential bonds. It also shows unpaired electrons which create a magnetic field and is a diamagnetic field. If there are paired electrons in the outer-shell, then it is diamagnetic. If there are unpaired electrons, it is paramagnetic.
Review: http://www.youtube.com/watch?v=Vb6kAxwSWgU
Ionization energy
The energy required to remove the outermost electron from a single atom. If the ionization energy is high, that means it takes a lot of energy to remove the outermost electron. The energy to remove an electron increases as you go across and up on the periodic table because of the increasing nuclear charge.
The largest atoms are on the bottom left while the smallest are on the top right because as the atomic number increases, the protons also increase, and the protons pull the electrons harder inward.