Intro to Physiology and Organic Molecules
Physiology is all about explaining the mechanisms of how the body works and understanding the mechanisms of action. Physiology will introduce you to dosage calculations as well which is very important for those of you going into nursing or pharmacy. You will also learn more about medications in physiology than any other class. In this introduction we’ll be talking about the stuff we are made of and what organic molecules actually are.
The Chemical Composition of the Human Body
- Water: 60%
- Protein: 17%
- Lipids: 15%
- Minerals: 5%
- Nucleic Acids: 2%
- Carbohydrates: 1%
The first thing listed is water and it says 60%. Water is the major chemical that we and all living things are made of. We are 60% of water, no surprise there.
Proteins come in second at 17%.
Lipids (aka fats) come in third and make up 15% of the body weight. Fats would still come in third, even for a skinny person. Why is that? That’s because you are made of trillions of cells and the cell membrane for each of them is made of phospholipid bilayers. So even if someone doesn’t have a lot of adipose tissue, 15% of their body is still going to be made of lipids.
Afterwards come minerals such as phosphate (PO4), calcium, potassium and sodium. These are also known as electrolytes because they have an electrical charge and are inorganic ions. After minerals are nucleic acids which are our DNA and RNA.
Surprisingly, the chemical we have least in our body are carbohydrates. Why that is somewhat surprising is because we eat carbohydrates all the time. Carbs are sugars, sugars are carbs. Even though we eat a lot of carbohydrates, the body uses them immediately and when we eat too much carbs, they are turned into fats. Only 1% of our body weight is made of carbs.
Organic Molecules
Organic molecules are hydrocarbon molecules. There are four major types of organic molecules: Proteins, Lipids, Carbohydrates and Nucleic Acids.
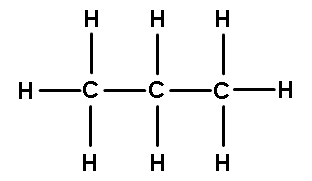
 Organic molecules are mostly made of chains of carbon atoms with hydrogen’s attached. On the right, we see the basic structure of an organic or hydrocarbon molecule (C-H-C-H). A carbon atom has four electrons in its outer orbit and these four valence electrons are shared with other atoms. The way we designate the sharing of electrons are with lines. These lines are called covalent bonds. The word covalent literally means “to share.” When these covalent bonds are broken, energy is released. So that is why when you have sugars or fats, when you break them apart, you release energy. Interestingly, breaking a hydrocarbon bond generates more energy than a carbon-carbon bond so these organic molecules contain a lot of energy.
Organic molecules are mostly made of chains of carbon atoms with hydrogen’s attached. On the right, we see the basic structure of an organic or hydrocarbon molecule (C-H-C-H). A carbon atom has four electrons in its outer orbit and these four valence electrons are shared with other atoms. The way we designate the sharing of electrons are with lines. These lines are called covalent bonds. The word covalent literally means “to share.” When these covalent bonds are broken, energy is released. So that is why when you have sugars or fats, when you break them apart, you release energy. Interestingly, breaking a hydrocarbon bond generates more energy than a carbon-carbon bond so these organic molecules contain a lot of energy.
Inorganic molecules are minerals, such as salt which is Sodium Chloride (NaCl), held together by an ionic bond. In general, ionic bonds are weak, so when they break they don’t release a lot of energy.
So that’s your introduction… now let’s delve deeply into the four classes of organic compounds…






