Firs t of all they’re going to give you information, periodic table, activity series of metals and they will give you solubility rules and formulas (like gas law, density, etc).
For the professors part, that’s going to be photocopied as well so you don’t have to memorize formulas and stuff. You have to spend time on nomenclature and concepts. The dept one is very heavy on moles.
Moles
Think about the various different ways that moles are used:
Grams to moles or vice versa (using formula weight)
Molarity times the volume: MV = n
Ideal gas law: PV/RT = n
You’ll have to deal with stoichiometry problems that may require you to get moles using the various ways above. Some questions may require you to balance the equation and then do the stoichiometry.
Classification of Reactions
How do you recognize combustion? Something reacting with oxygen. Lots of times those are organic products and the products are CO2 and Water.
How do you recognize a single displacement? One individual substances + another thing you could exchange it with. Then you have to think if it would proceed. You need to know the activity series (the more reactive species ends up in the ion form, so copper vs zinc, zinc ends up as zn+2 and copper ends up as a (s) solid)
How do you recognize double displacement situations?
It’s most commonly two salts. A positive ion and negative ion on one reactant and the same for the other reactant. And if there’s a reaction you could just switch the positives. To decide if it’s going to produce as a reaction, look at the two new combination of ions and see whether either of them are insoluble. If it is insoluble, it would end up as precipitate and read as (s) in the equation indicating solid.
Synthetic reaction: You end up with fewer things than you started with
Decomposition reaction: You end up with more things that you end up with.
Redox reaction: If you see a change in the charge, it’s an indication of a redox reaction.
Electron Configuration
You will be given an element and asked what’s the electron configuration of it.
For instance if they give you cobalt. They would give you many possibilities but the correct one would be Co = [Ar]4s2 3d7 (fourth row row of transition metals, and 7 positions over in the column) the first two elements, potassium and calcium with s type orbitals. in the transition orbitals it’s 7 positions over so there would be 7 electrons in the d orbital.
STP
Standard Temperature and Pressure is 273K and 1.0atm. It’s used because it’s a useful conversion factor, because at STP you have 22.4 L/mole, or 1 mole per 22.4 Liters. It’s a useful conversion factor.
Dot Structures
They’ll give you a formula and you’ll have a choice of choosing dot structures. Remember if there’s resonance, the correct choice would be the one that shows the resonant structures.
So let’s say you are presented with CO3– You would have how many resonant structures? Three because there’s 3 different places you could place the double bond. So the proper choice would be the one that shows all three resonant structures.
Remember halogens (Group 17) can never have double bonds. So if they are asking about resonant structures and it involves halogens, there is no resonance.
Titrations
Remember that a titration involves adding a solution to get to an end point. At the end point of a titration, the moles of the acid equal the moles of the base (na=nb). Moles is a product of the molarity times the volume in liters for either the acid or the base.
MaVa=na=nb=MbVb
If the base has only one hydrogen ion and the acid only has one, then you could use the formula like this. However, if you’re dealing with 0.2M of sulfuric acid (H2SO4) of it, you’re concerned with the hydrogen ions (2H+) so you want to write the molarity of it at 0.40M when you use it for Ma because there’s two hydrogens.
You could even have Phosphoric Acid (H3PO4), so whatever the molarity is, it’s multiplied by 3 due to the three hydrogen ions.
As for a base, concentration of hydroxides in Barium hydroxide (Ba(OH)2) would be 2 times.
Balance equations
Learn to balance equations.
Memorize the Gas Laws
Combined gas law: when you have a fixed amount of gas and just changing variables (PV/T=PV/T). You could drop out a variable if there’s no indication of change.
Ideal gas law: PV= nRT
R has the value 8.314 J·K−1·mol−1 (equal to the product of Boltzmann’s constant and Avogadro’s constant.)
Density
Remember the density experiment in the lab. We have water in a graduated cylinder. We check the volume level. Then we put aluminum pellets in there. This increase of volume of water once you put the object in there can help us figure out what the density of the object (aluminum) is.
Significant Digits
They will be a real stickler on significant digits. That will include reading a volume off a graphical graduated cylinder, read the bottom of the miniscus. If it’s the bottom of the line, it’s going to be 0. If it’s half way or between the line it’s going to be 0.5 or 1.
You’re almost certain to get a question regarding adding, multiplying and dividing to see if you get the right significant digits as a result.
For example, (31.6 – 30.5) x 612.4 / 3.00 x 105
Do the first thing in the parenthesis so that’ll be 1.1, multiply that by 612.4 and divide it. They may give you the answer or they may ask you how many significant digits are in this? And in this case it’s two.
Always keep in mind when you’re adding and subtracting, the least far to the right you could go is the most you can go and if they ask for significant digits just count over to that position from the dot.
Know how to recognize oxidizing and reducing agents. In a redox reaction know what is the reducing half and oxidation half.
Isotopes
Isotopes are atoms of the same type (same # of protons) but they may have a different number of neutrons. Suppose you have 56Fe, they could ask you, how many protons, how many neutrons, how many electrons are there?
The number up there (56) is the mass number (protons + neutrons). You could tell what the protons are by looking at the periodic table (26). Together it’s 56, so that’s a difference of 30 which are the neutrons. In a neutral atom the protons are equal to the electron so they would be 26 electrons.
They could also give you 56Fe++ in this one you’re losing 2 electrons so the electrons would be 24.
VSEPR
This is the model for predicting shapes. Remember the procedure: Do the dot structure first. Figure out the geometry of electron sets. Look at how many electron sets you have (2 = linear, 3 = trigonal planar, 4 = tetrahedral). As for the shape, look where things are attached.
Molecular Polarity
Look for all the polar bonds and once you get the shape of the molecule look if there’s a net pull of electrons in one direction. Remember it’s pretty much a diagonal line up to Fluorine, not counting the Noble gases. Fluorine is the most electronegative.
PH Problems
Example 1
What is the Ph if you have a concentration of hydrogen ions given like this:
[H+] = 1.0 x 10-5 MTo get the Ph, you take the exponent, and change the sign, so the Ph will be 5.
Example 2
[OH-] = 1.0 x 10-11MIn this one, you have to get the concentration of hydrogen ions. the hydrogen ion concentration os 10 to the -14 so get the difference would be a Ph of 3.
Example 3
Suppose they give you an example where the thing is not 1.0 such as:
[H+] = 4.6 x 10 -8You could determine exactly what it is on your calculator or estimate it. 4.6 is greater than 1, so you could imagine it’s between 1.0×10-8 and 1.0×10-7. You know what the PH of these two is.
1.0 x 10-8 = PH of 8
1.0 x 10-7 = PH of 7
On your calculator, what you do is enter 4.6. press exponent. then press 8. change sign. So this comes out to 7.34.
Nomenclature
Chances are they will give you formulas and maybe options for what the name is for that formula and it could be, say an ionic compound or a molecular compound. Remember if it’s ionic, there’s some ions you need to think of to specify the charges, so if it’s Cu(NO3)2. What would be the name? Copper (II) Nitrate.
Remember covalent compounds. Know the prefixes of those.
Which acids will be used with the hydro- prefix? Whenever the negative part of an element ends in -ide, so the simple ions like chloride for example. The exception to this is cyanide.
Hydrates just indicate the number of waters you have, so for example hexahydrate indicates 6 of them.
Diagram type problem
Imagine one empty circle that represents an element and one shaded circle that represents another. And those two together, represents a compound. You may be presented with a picture of boxes with varying amounts of these compounds or separate elements. Which would have the higher equilibrium constant? The one with relatively more products than reactants.
Imagine energy levels spaced out labeled N1, N2, N3, N4, N5. N1 would be like a ground state and it could jump up to N5 let’s say. The first jump is much bigger than the second and with each level, they are very rapidly getting compacted. So they may ask you which one that jumps down gives off the most energy?
Remember energy is inversely related to wavelength.
The highest energy would be the largest jump.
The largest jump corresponds to the shortest wavelength.
When things are moving down, they are giving off energy.
So if you are given choices of 5->3 and 3->2, and 4->1 just look at the distance between them to find . Which of them is going to give the longest wave length?
 =
= 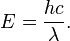
Organic chemistry
Memorize: Alcohol, Aldehyde, Ketone, Ether, Amine, Carboxylic Acid. They may give you several structures and ask which one is the aldehyde.
What happens when you have evaporation? There’s a cooling effect. The most energetic molecules are the ones that are leaving, leaving behind a lower average kinetic energy and lowering the temperature and there’s a cooling effect.
Equilibrium
Support you have a system that’s coming to dynamic equilibrium. Once you’ve reached there, what’s happening? You equalize the rates of going forward and backward and the concentrations stay the same.
Empirical Formulas
The idea is to get the simplest ratio of components of moles in whole numbers. So you could have weight composition or percent composition. If you’re given info in a percent composition, assume 100 grams, then you could immediately convert those percentages to grams, then you’ll have a composition situation.
Take the grams of the various components. Multiply them by their atomic weight. You’ll get moles of each of them. Then after that, you scale them until you can get them to whole numbers. Typically divide the lowest by itself and the others by that number. Then you may have to multiple it by a factor to get it at a whole number.






