The Resting Membrane Potential
We’re going to deal with electricity because we’re going to be talking about the electrical properties of cell. So first let’s get acquainted with some basic electrical concepts.
Basic Electrical Concepts
1) There are positive charges and negative charges. What is the origin of these charges? We have protons and electrons. We know that if there is a difference in either two, there is a polarity. Even in a large molecule like a protein molecule, if the electrons exceed the protons, then that molecule has a slightly negative charge.
2) Two like charges repel.
3) Opposite charges attract.
Electrical potential (voltage)
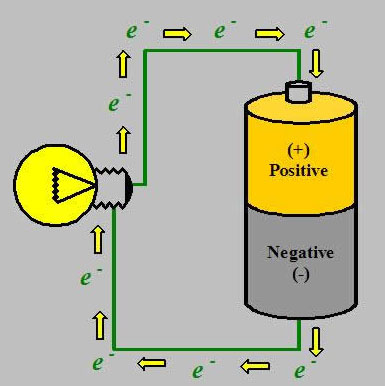 Everyone knows one end of the battery is positive and the other end is negative. The reason why it’s like that is because it’s constructed with chemicals inside. The chemicals that make up the battery on the positive end have an excess of positive charges. The chemicals on the negative end have an excess of negative charges on that end . These chemicals don’t want to be together (because like charges repel each other) but that’s the way it’s constructed. We could take a voltmeter (battery tester) and put an electrode (probe) to one end of the battery and another to the other end. What this tells us is the difference in electrical polarity. The greater the difference of electrical polarity, the greater the voltage. Voltage is synonymous with electrical potential and both mean a difference in electrical polarity.
Everyone knows one end of the battery is positive and the other end is negative. The reason why it’s like that is because it’s constructed with chemicals inside. The chemicals that make up the battery on the positive end have an excess of positive charges. The chemicals on the negative end have an excess of negative charges on that end . These chemicals don’t want to be together (because like charges repel each other) but that’s the way it’s constructed. We could take a voltmeter (battery tester) and put an electrode (probe) to one end of the battery and another to the other end. What this tells us is the difference in electrical polarity. The greater the difference of electrical polarity, the greater the voltage. Voltage is synonymous with electrical potential and both mean a difference in electrical polarity.
Resistance and Electrical Current
The charges inside of a battery cannot move because there is a highly-resistant separator that prevents them. internally. Air is also a great resister, meaning it hinders the flow of current. If we take a copper wire, which has a low electrical resistance that doesn’t hinder the movement of electrical charges, the positive charges are always going to move to the negative charges. When these charges start moving, that’s an electrical current. The lower the resistance, the greater the current. In reality, protons don’t move, electrons do. What’s moving in the wire is not positively charged electrons but negatively charged ions going from negative to positive. Eventually when we no longer have this difference in electrical polarity, the battery will be dead.
Ohm’s Law
So far we’ve described voltage, electrical current, and resistance. Now we tie those in with Ohm’s law, described by Georg Simon Ohm in 1827.
Current (I or C) = Voltage / Resistance
C = V/ R
The cute thing about these little formulas is that if you know any two of these you could solve for the third.
The Resting Cell Membrane Potential
The year is 1942, two British electrical engineers, Hodgkin and Huxley become the first people ever to record the electrical signal of a cell. It was a neuron from a squid. They had this cell in fluid and a voltmeter just outside of it. They were able to construct a microelectrode, a probe so small they could attach it to the cell without killing the cell. They wanted to see if there was a difference in electrical potential (voltage). They found the inside of the cell had an excess of negative charges and outside the cell has an excess of positive charges. This difference in electrical charges exists in the cell membrane. What do we call it when we have a difference in electrical polarity? Voltage.
This voltage is found in every single cell of every living thing. So this isn’t just in the nerve cell. It’s found in plant cells, bacteria cells, and so forth. All cells exhibit an electric potential (voltage) called the “Resting Cell Membrane Potential.” They measured this voltage and found it varies in cells from 70-90mV, usually it’s written as -70mV or -90mV because it’s a negative voltage.
So, okay, a thousand milliliters is one liter, right? A thousand millivolts is one volt. If the voltage in the cell is, say, let’s round it to 100mV, what is the voltage of the cell in volts? A tenth of a volt.
1,000mL = 1L
1,000mV = 1V
100mV = 0.1V
To relate to this, the most common batteries we use are 1.5 volts. So a cell is less than a tenth of a volt. How many cells are in your body? Sixty trillion cells and each one is like a battery. The amount of electrical energy in your body is AWESOME.
Ever seen “The Matrix” movie? What were the machines that have taken over the world using the humans in the incubators or? As batteries to power the machines. Every single cell in your body is a battery and you have 60 trillion of these cells. Electroencephalography (EEG) monitors the electrical energy of your body, electrocardiography (ECG) for your heart, electromyography (EMG) for your muscles and so forth.
Where does this voltage come from?
We’re going to introduce this in three steps. When you learned intracellular fluid and extracellular fluid we said that there was a lot of Potassium (K+) inside the cell and Sodium (Na+) outside the cell in the ECF. And remember in the ICF we also have tons of proteins which don’t exist in the tissue fluid or blood plasma and they happen to be slightly anionic. The difference between the Na+ outside the cell and the K+ & Proteins inside the cell are what create the difference in electrical polarity.
How come almost all the potassium is inside of our cells and all the sodium is outside?
Because there’s a Sodium-Potassium ATP pump that concentrates the potassium on the inside and the sodium on the outside.
The most important numbers to know are…
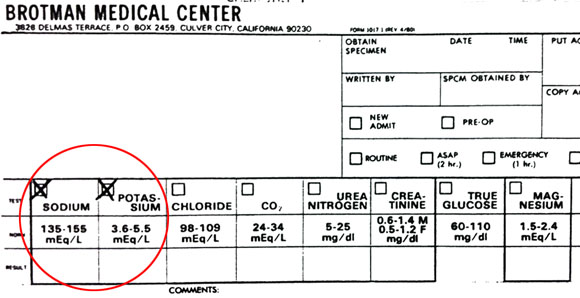
[Na+]ECF = 150 mEq/L[K+]ECF = 5 mEq/L [Na+]ICF = 15 mEq/L
[K+]ICF = 150 mEq/L
Clearly the majority of the potassium is on the inside. These concentrations are in milliequivalents per liter because electrolytes are always expressed in milliequivalents.

If you think this has nothing to do with nursing, look at this chart above stolen from the Brotman Medical Center hospital. If you’ve worked in a hospital you know they’re always checking your electrolyte levels. The normal amount of sodium is listed as 135-155mEq/L and potassium is listed as 3.6-5.5/mEq/L. We are just rounding them off to 150 and 5 to keep things simple. When these change, it affects the electrical activity of all our cells.
Do all molecules affect the electrical polarity of the cell?
If oxygen flows into the cell, does that change the electrical polarity of the cell? No, because it is electrically neutral. If CO2 is going down its concentration gradient, it doesn’t affect the electrical polarity of the cell either because it’s electrically neutral. If glucose goes in and out of a cell, it doesn’t affect it either because it’s not an ion or electrolyte and has no electrical charge If water goes in or out of the cell it doesn’t affect it either.
Physiological Basis of the Resting Cell Membrane Potential
There’s a high concentration of potassium in the cell. There’s also various negative charges in the cell with the major negative charge being the proteins. The major positive charges outside the cell is sodium (Na+).
The cell membrane is often described as being semi-permeable which means some things could get through and some cannot. Remember we talked about ion channels embedded in the membrane. All of the ion channels are normally closed with one exception: Potassium. Cell membranes are normally permeable to K+ but not to Na+ or any other electrolyte. Chloride would like to flow but chloride ion channels are closed. Proteins are too large to flow through any ion channels.
Why does potassium flow out of a cell? Because of a concentration gradient. The normal potassium levels are 150mEq/L inside the cell. As positively-charged potassium (K+) flows out of the cell, that’s going to make the inside more negative and the outside more positive. As K+ diffuses out of the cell due to the concentration gradient, the inside of the cell becomes negatively-polarized.
Why doesn’t potassium flow out of the cell until there are equal amounts inside and out?
Every time a potassium diffuses out, it makes the difference in electrical polarity stronger. Normally chemicals diffuse until their concentrations are equal everywhere. So we would expect the potassiums to have diffused until potassiums were equal inside and out, but that’s not going to happen because there’s also an electrical gradient. We don’t talk about electrical gradients with molecules that have no electrical charge, like with glucose, but when we talk about a diffusion of something with an electrical charge, there is a concentration gradient and an electrical attraction gradient.
As potassiums flow out due to the concentration gradient, the inside of the cell becomes more negative, which attracts the potassium (K+) back inside the cell because opposite charges attract and the potassium ion channels are always open. When the electrical gradient is pulling something in is as strong as the concentration gradient pushing it out, then it reaches equilibrium. The electrical polarity on the inside is about 70-90mV and it’s strong enough to keep the potassiums in.
This is occurring because only potassium ion channels are open. If sodiums were able to move in, then this wouldn’t happen, but remember potassiums are the only ones that can move out of the cell. This is what creates the electrical polarity.
1) The concentration gradient causes K+ to diffuse out of the cell.
2) The electrical gradient that develops, causes K+ to diffuse back into the cell.
This equilibrium (steady-state) potential of 90mV is called the resting cell membrane potential (voltage) because it’s the difference in electrical polarity across that cell. What’s creating this voltage is the fact that only potassium can flow through the membrane.
What about the sodium potassium ATP pump? Is that going to change anything? No. At the simplest level, if all the pump did was to kick a positively charged sodium out and bring a positively charged potassium in exchange, then nothing would change. And if it pumped a potassium into the cell, the concentration gradient would increase causing another potassium to diffuse out of the cell. Thus, the membrane potential tends to remain constant.
Calculation of the resting cell membrane potential using the Nernst Equation
The Nernst Equation can predict the voltage of any cell as a battery. This may help somebody understand.
Vmembrane = RT/zF * ln(C1/C2)
- RT = Boltzmann Constant
- z = Valence (+1 for K+)
- F= Faraday’s constant is 96485 Coulombs per mole
- ln(C1/C2) = logarithmic difference between inside (C1) and outside (C2)
Remember what is the normal amount of K+ on the inside? 150mEq/L. And on the ouside is 5mEq/L. What’s the difference between the two? 30. On your calculator if you hit 30 and log you get 1.477. If you multiply that by 60 (which is RT/zF), you get 88.6mV.
When the K+ electrolyte levels change, the voltage for every single cell of your body changes.
Next, we explain why this subject is important clinically…






