Chemistry Part 6
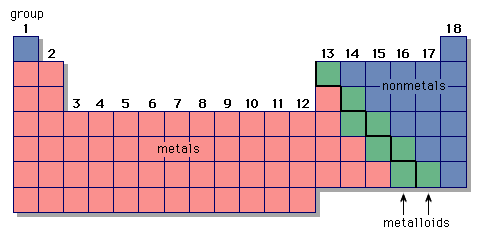
 Reactivity Series of Elements
Reactivity Series of Elements
The coinage metals are the ones with the lowest reactivity. The reactivity series gives an idea as to which element is capable of displacing other elements in a displacement reaction. Here’s a mnemonic to help you learn the reactivity series:
Private Second Class Mac Zitl He Can Make Some Gun Powder
Private (potassium)
Second (Sodium)
*Class (Calcium)
M (Magnesium)
a (aluminum)
c (Carbon)
Z (Zinc)
i (iron)
t (Tin)
l (Lead)
He (Hydrogen)
Can (Copper)
Make (Mercury)
Some (Silver)
Gun (Gold)
Powder (Platinum)
Li, K, Ca, Na, Mg, Al, Zn, Cr, Fe, Cd, Ni, Sn, Pb, H2, Cu, Ag, Hg, Au
Evidence of a reaction
- Precipitate: when you join 2 things and a solid forms.
- Change in appearance of the surface of the material.
- In there is a gas given off.
- Color change.
- Change in temperature.
Single Displacement
A + BC -> B + AC
2 Ag NO3 (aq) + Ca (s) -> Ca(NO3)2(aq) + 2Ag (s)
Zn(NO3)2 (aq) + Cu (s) -> NO REACTION because Zn is more reactive than copper. The more reactive metal ends up with a charge.
Double Displacement
AB + CD -> AD + CB
The most common reaction is precipitate in a double displacement reaction. Two solutions that are soluble could become solid. In an acid-base reaction one product is water because the acid has hydrogen ions (H+) and the base has OH- so H+ and OH- = H2O + “salt” + heat.
Solubility Rules
1. Soluble: Group 1 alkali metal compounds, acetates, nitrates and ammonium compounds are all soluble.
2. Soluble: Hydroxides of Group 1 alkali metals and NH4+1, Ca+2, Sr+2, and Ba+2 are soluble. All others are insoluble.
3. Soluble: Group 7 halogens/halides are soluble except for those containing Ag+1, Pb+2, and Hg2+2.
4. Soluble: Most sulfates (SO4) are soluble, except for BaSO4, SrSO4, Ag2SO4, PbSO4 and CaSO4. In general, if it’s a sulfate and it’s not with one of those Ba, Sr, Ag, Ca, Pb, then it’s aqueous.
5. Insoluble: Most phospates, carbonates, chromates and sulfides are insoluble (except those of the alkali metals and ammonium).
Electrolytes
Examples of strong acids are hydrochloric acid (HCl), hydroiodic acid (HI), hydrobromic acid (HBr), perchloric acid (HClO4), nitric acid (HNO3) and sulfuric acid (H2SO4).
Examples of strong bases are are Group 1 Hydroxides and Group 2 Hydroxides (except Mg (OH)2)






