Chemistry Part 3

The reason several elements have different symbols on the periodic table than their name is because they used to be called by their latin names.

Origins of Latin or Latinized Greek element symbols
| Chemical | Symbol | Latin Name |
|---|---|---|
| Copper | Cu | Cuprum |
| Gold | Au | Aurum |
| Iron | Fe | Ferrum |
| Lead | Pb | Plumbum |
| Mercury | Hg | hydrargyrum (hydr=water argyros=silver) |
| Potassium | K | Kalium (alkali) |
| Silver | Ag | Argentum (arg=grey or white) |
| Sodium | Na | Natrium |
| Tin | Sn | Stannum |
| Tungsten | W | Wolfram |
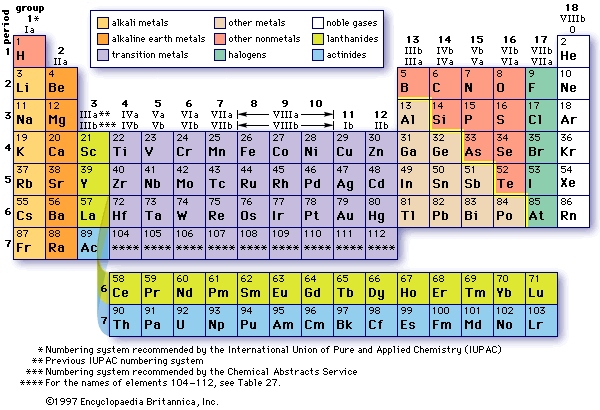
Properties of Elements
Metals = Malleable, conductor, luster
Nonmetals = Brittle, nonconductor, no luster
Metalloids = Have properties in between (semiconductors)
The metals tend to lose electrons. The nonmetals tend to gain electrons.
The periodic table was created by Dmitri Mendeleev. He got credit for it because he acknowledged elements that were undiscovered and very accurately predicted their tendencies.
Properties of Elements on the Periodic Table by Location
Left side of Periodic Table = Reactive metals / most reactive.
The second last column are the second most reactive.
The metalloids are along the jagged edge except for Aluminum.
Transitional elements have intermediate reactivity (in the middle).
The noble gases have the least reactivity (last column).
The precious metals such as Gold, Silver, Platinum and Palladium are the second least reactive group as well.
Groups refer to columns while Periods refer to rows.
Isotopes
There can be 1-5 isotopes of an element but there are usually 1 or 2.
Chlorine has two isotopes. 35Cl and 37Cl
| isotope | mass(amu) | % | fractional | |
|---|---|---|---|---|
| 35-Cl | 34.97 | 75.53% | x 0.7553 | = 26.41 |
| 37-Cl | 36.96 | 24.47 | x 0.2447 | = 9.04 |
| Average | mass of | weight | = 35.45 amu |






