Chemistry: Gases, Liquids, Solids
Solids tend to create a crystalline structure while liquids are free to move around each other but still remain together. Gas molecules are separate from each other.
| Solids | Liquid | Gases | |
|---|---|---|---|
| Density (g/cm3) | high | high | very low (g/L) |
| volume/shape | fixed | fixed/container | indefinite/container |
| Compressibility | very low | very low | high |
Properties of Gases
- Low Density
- Expand to fill container
- Easily compressed
- Exert pressure
- Leak through small holes
At sea level the height of a mercury tube will be 760mm of Hg. This is also known as the Torr.
760mm Hg = 14.7 psi = 101.3 kPA = 1.0 atm
Device that measures external pressure is a barometer and one that measures internal is a manometer.
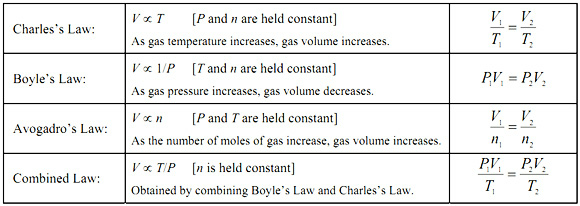
Charle’s, Boyle’s, Avogadro’s and Gay-Lussacs Laws

In Charles law, if pressure is constant, Volume and Temperature have a linear relationship. It took a long time to figure this out because thermometers didn’t exist and Kelvin wasn’t established yet to do away with negative numbers. V=kT or V/T=k
Are you could see from Boyle’s law, V=k(1/P) or PV=k, volume and pressure have an inverse relationship.
Gay Lussac’s law or pressure-temperature law states that PT=k
If we combine all those we get that PV/T=k, which means that we get the combined gas law (above).
Pressure and Volume can be in any unit but temperature must always be kelvin.
Avogadro’s law helps us know that V/n=k or V=kn which helps us create the ideal gas law.
PV=nRT
Standard Temperature and Pressure = STP = 273K and 1.0atm.
Daltons law of partial pressure deals with gas mixtures. Each of these particles exert a certain amount of pressure so the formula would be like PT = P1 + P2 + P3 + … This is useful for figuring out a mixture such as air which consists partly of Oxygen, Nitrogen, Argon and so forth and each of these elements exert a unique amount of pressure.
Kinetic Molecular Theory (Maxwell, ~1870)
1. Molecules are in constant, rapid, random motion. (Air molecules travel at 900mph)
2. Negligible volumes and interactions between molecules.
3. The average kinetic energy (KE = 1/2 mv2) of the molecules is proportional to the Kelvin temp.
Liquids
Intermolecular forces are those forces between substances.
- Very strong = solid such as ionic compounds that have charge and EM force.
- Intermediate strength = liquid at room temp and strong enough to be together.
- Weak = Gas
Temperature opposes these forces. Melting = fusion.
Sublimation: Directly going from solid to gas (like CO2 which is dry ice). Deposition is gas to solid.
Polar Molecules
Trends: If you attach two of the same elements, they create higher melting points.
All the H2‘s have higher melting points than predicted (H2O, H2, H2se, H2Te) because of their especially strong dipole-pole attractive forces.
Hydrogen bonds: Hydrogen bonds are the intermolecular forces between hydrogen and electromagnetic elements of other molecules, only when the hydrogen is bonded to Fluorine, Nitrogen and Oxygen. F-H, O-H, N-H have especially high intermolecular bonds.
As you move down the table for halogens, the intermolecular bonds increase: F2 = gas, Cl2 = gas, Br2 = liquid, I2 = solid
London dispersion forces. Initially everything is symmetrical, when the electron moves to one side, for a brief period of time it will act like a polar molecule b/c one side is slightly more positive. This is happening all the time.
Properties of liquids
- They evaporate because of kinetic energy
- Temp increases evaporation rate
- Surface area increases evaporate.
- Air flow increases evaporation rate.
Rubbing alcohol feels cold cause of the fast evaporation rate. Water feels cold on a windy day cause it’s evaporating.
Vapor Pressure
Vapor pressure is the pressure of a vapor in contact with its liquid or solid form. Alcohol, like all volatile liquids and organic solvents, has a very high vapor pressure. Mercury has a low vapor pressure.
Boiling Point
Little bubbles that come up during boiling are air that are dissolved in the water. The higher the temperature, the more that comes out. Eventually you have big bubbles during boiling. Water is becoming gas and outside we have atmospheric pressure.
The larger the bubbles are, the lower the atmospheric pressure. The higher the atmospheric pressure, the smaller the bubbles.
Boiling occurs when the inside vapor pressure exceeds the atmospheric pressure. You could adjust the boiling point by adjusting the pressure. What happens when you go to high altitudes? Boiling point gets lower. At extremely high altitudes, water boils at 70 degrees which is not high enough to cook food with. The opposite scenario is the pressure cooker. It creates higher atmospheric pressure which means the water boils at a higher temperature and will cook the food faster.
Viscosity is the resistance to flow. Shape of molecules determines. Spherical molecules move easily while irregular shapes are harder. The most viscous molecules are DNA because they are very elongated.
Capillary Action. Water is strongly attracted to the glass. Stronger than it is attracted to other water molecules. This causes it to go up in the tube. The thinner the glass is, the higher the surface area so it pulls up more. Mercury actually goes lower than the glass because the attractive force is stronger toward mercury than glass. Adhesion is the force at the surface between other substances. Cohesion is the force within the same liquid. A bug can stand on top of water because of the forces of cohesion. Water rises up a glass tube because of forces of adhesion.






