Acids and Bases
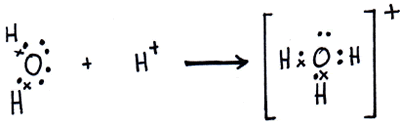
As you may know, hydrogen ions (H+) are found in acids and hydoxide ions (OH–) are found in bases.
Arrhenius in the 1890’s made the following discovery:
- Acid generates H+ ions in aqueous solutions.
- Base generates OH– in aqueous solutions.
Bronsted-Lowry came up with another definition you may have never heard before.
- Acid is a proton donor.
- Base is a proton acceptor.
So let’s see how that correlates with our original understanding.
If we have HCl -> it dissolves into H+ + Cl–
If we have NaOH -> it dissolves into Na+ + OH–
A hydrogen ion gets donated to a hydroxide ion and forms water: H+ + OH– -> H2O
Another definition that is also good to know is… Consider ammonia..
If we look at NH3, we can’t tell, but if we put it in water, what do we get?
NH3 + H20 -> NH4OH4. We get sodium hydroxide. There will be some ammonia ions separated into hydroxide ions and we get a basic solution.
Let’s imagine we have gaseous ammonia and gaseous hydrochloride. What will happen is this will get transformed into ammonia and chloride, a salt. One is a base and one is an acid and this is an example where ammonia would be considered a base in an aqueous solution.
NH3(g) + HCl (g) -> NH4Cl
So let’s imagine we have hydrochloric acid, and let’s imagine we have acetic acid and also sulfuric acid. Let’s do dot structures on these.
HCl. ![]() The hydrogen is a weak bond. This ionizes 100%. (strong acid)
The hydrogen is a weak bond. This ionizes 100%. (strong acid)
HC2H3O2 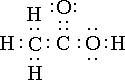 In the formula we see 4 hydrogens but only one of them are in the front. That is because only one of them is weak in the dot structure. Those 3 hydrogens are never going to break off. This ionizes less than 5%.(weak acid)
In the formula we see 4 hydrogens but only one of them are in the front. That is because only one of them is weak in the dot structure. Those 3 hydrogens are never going to break off. This ionizes less than 5%.(weak acid)
H2SO4 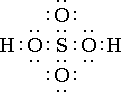 This ionizes 100%.(strong acid)
This ionizes 100%.(strong acid)
Strong acids dissolve 100%. Ionic compounds, salts, do this, such as sodium chloride, separate into sodium ions and chloride ions.
Weak acids only partially ionize, maybe 2% or 5%. That means most of the molecules stay as the acid.
Memorize the strong acids
HCl3, HBr, HI (you could think of this as a group because hydrogen attaches to a halogen, except fluorine)
HNO3
H2SO4
HClO4
The rest are weak.
Memorize the bases
Strong bases: Group 1 hydroxides and Group 2 hydroxides, except, Magnesium Hydroxide (MG(OH)2).
Weak bases ones are the ones with limited solubility, such as ammonia, NH3, is weak.
Notice these hydroxides act like salts, they dissolve and separate into ions, like all salts do. Same way with hydroxides.
Mg(OH)2 -> Mg+ + 2OH–(g)
Magnesium hydroxide is not very soluble. It has limited solubility, meaning not very much is going to dissolve. You get low concentrations of hydroxides. It will act like a weak base in that it doesn’t generate a high concentration of hydroxides.
NaOH –> Na+ + OH–
Sodium hydroxide is very soluble.
If a hydroxide is soluble, it will generate a high concentration of solubility, so it will be a strong electrolyte. If it is not soluble, it will be a weak electrolyte.
A base must always has a free pair of electrons to attach to.
Always when a base is accepting a hydrogen ion, it’s doing so by having a free pair of electrons for it to attach to. It’s necessary but not sufficient though.
For instance, we have water.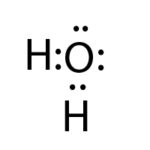 Think it could attract a hydrogen ion?
Think it could attract a hydrogen ion?
Yes it does.

Remember, hydrogen is just like a proton because it has only 1 proton and 1 electron and if the electron is taken away, it’s just a proton. If you put it into water, it is immediately going to attach to a water molecule. They always attach to water and exist like that. A base always has a free pair of electrons, but not everything that has a free pair of electrons, is a base.
Conjugate Acid-Base Pairs
Let’s say you have hydrochloric acid and it separates into H+ and Cl- ions. When it does that, it’s acting as an acid.
HCl (acid) -> H+ + Cl– (base, b/c conceptually it can go back)
Now let’s suppose we start with H+ + Cl– and it’s becoming HCl. In this case, Cl is acting as a base because it’s accepting a hydrogen ion. There is very little tendency to associate and come back to this but Cl conceptually is acting as a base:
H+ + Cl– (weak base) -> HCl (strong acid)
So whenever an acid generates a hydrogen ion it’s counterpart is a base. These bases are called Conjugate Bases. So chloride is the conjugate base of hydrochloric acid. F– is the base for hydroflouric acid.
HF (weak acid) -> H+ + F– (strong base)
H2O + F– (strong base) -> HF (weak acid) + OH–
There is an inverse relationship of strength.
(strong acid) HCl -> H+ + Cl– (weak base, b/c its chances of going back is low))
H+ + Cl (base)-> HCl (weak base because it has little tendency to go left)
HF (weak acid) -> H+ + F– (strong base)
H2O + F– (strong base) -> HF (weak acid) + OH–
| acid | conjugate base |
|---|---|
| strong acid | weak |
| weak acid | strong |
So when these 2 interact, the strong base dominates.
Toxicity
With certain ions you have to be careful. This combination produces carbon dioxide.
H+ + CO3— ->H2CO3 -> H2O + CO2 (g) This combo isn’t toxic.
H+ + S— -> H2S (smells terrible, and is toxic) If you make this solution acidic, you’re going to present a gas and make a hazardous situation.
H+ + CN– -> HCN (g) Hydrogen cyanide gas is not just toxic, it’s deadly. They take NaCN and throw it in an acid and generate hydrogen cyanide gas.
Let’s pretend you have sulfuric acid of H2SO4. Take a solution of sodium hydroxide NaOH and put it on there. What happens if you put them together? Remember strong bases are just as dangerous as strong acids. So what you want to do is put it in a weak base such as sodium bicarbonate NaHCO3 that will neutralize all the acid. It will dissolve an acid just fine without creating any problems. Another characteristic of this… if you put it in the water, you will generate sodium ions and bicarbonate ions (Na+ + HCO3–). Let’s think of HCO3– whether it is an acid or base. It is actually amphoteric because it can act as both an acid or base.
It can act like this as an acid: HCO3– -> H+ + CO3—
Or it can accept a hydrogen and act as a base: H+ + HCO3– -> H2CO3
These can act as buffers in nature to keep a certain pH.
Water is also amphoteric:
H2O -> H+ + OH–
H+ + H2O -> H3O+
Neutralization Reactions
So we talked about different classes of reactions. This is going to be an acid/base reaction. The simplest kind you could have is this:
HCl + NaOH -> NaCl + H2O (sodium chloride and water)
HC2H3O2(acetic acid) + NaOH –> NaC2H3O2 + H2O (now this is the salt that’s generated, remember salts are just ionic compounds). When this is an acid base reaction, the molar ratio between each other is 1 to 1.
But what if you have this reaction?
H2SO3 (weak acid) + 2NaOH (strong base)
(in this one you have 2 hydroxides when you balance it, and it’s going to require a 2:1 molar ratio for the reaction to be balanced. It doesn’t matter if one is strong or weak).
Ma = Mb (moles of acid = moles of base)
MaCa = (Ma = Mb) = MbVb (now we simplify this)
MaVa = MbVb Used for titrations: add relative amounts until you get the desired neutralization and then you can calculate the concentration of something.
We could put a compound that will turn pink when something turns to basic. SO if you’re adding a base to an acid, it will eventually become basic. When it becomes basic, you calculate how much base you added and then figure out the concentration.






